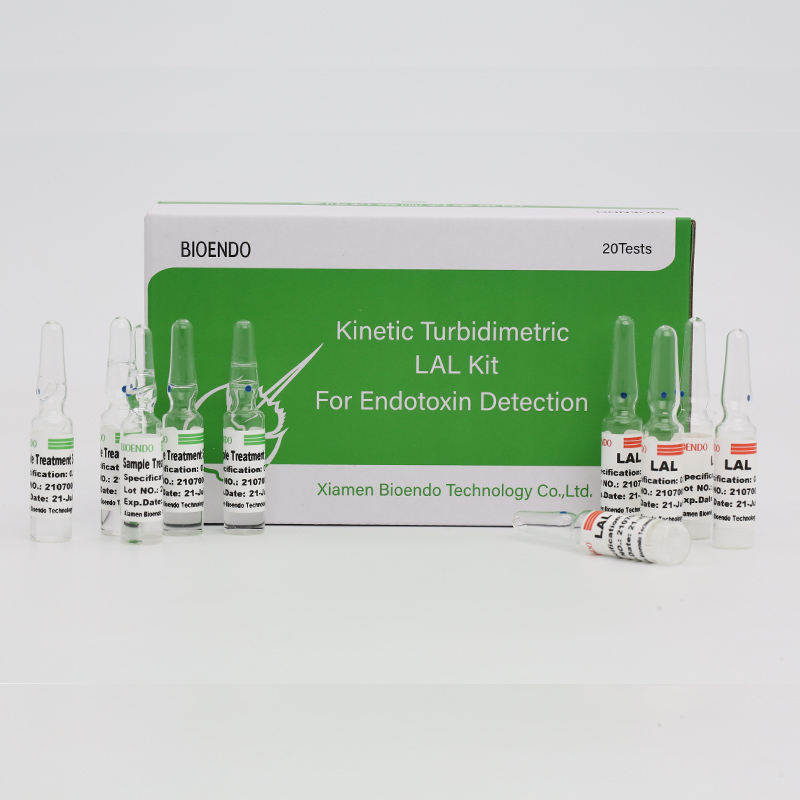
Ikhithi yokuHlola ye-Endotoxin yePlasma yabantu
Endotoxin Assay KitkwiPlasma yoMntu
1. Ulwazi lweMveliso
I-CFDA isusiweIkiti yovavanyo lwe-Endotoxin yonyangoUbalula inqanaba le-endotoxin kwiplasma engenabuntu.I-Endotoxin yinxalenye enkulu yodonga lweseli ye-Gram Negative bacteria kwaye yeyona nto ibalulekileyo ye-microbial mediator ye-sepsis.Amanqanaba aphezulu e-endotoxin anokubangela umkhuhlane, utshintsho kwinani leeseli ezimhlophe zegazi kwaye, kwezinye iimeko, ukothuka kwentliziyo.Isekelwe kuvavanyo lwe-Cpathway kwi-limulus Polyphemus (igazi loononkala behashe).Ngomfundi we-kinetic microplate kunye nesoftware yokuvavanywa kwe-endotoxin, ikhithi yokuvavanya i-Endotoxin ibona inqanaba le-endotoxin kwiplasma yomntu ngaphantsi kweyure enye.Ikhithi iza ne-plasma pre-treatment reagent ephelisa izinto ezithintelayo kwi-plasma ngexesha lovavanyo lwe-endotoxin.
2. IParamitha yeMveliso
Uluhlu lovavanyo: 0.01-10 EU / ml
3. Uphawu lweMveliso kunye neSicelo
Iza kunye nezisombululo ze-plasma pretreatment, isusa izinto ezithintelayo kwiplasma yomntu.
Phawula:
I-reagent ye-Lyophilized Amebocyte Lysate (LAL) eyenziwe yi-Bioendo yenziwe nge-amebocyte lysate ethathwe kwigazi loononkala.
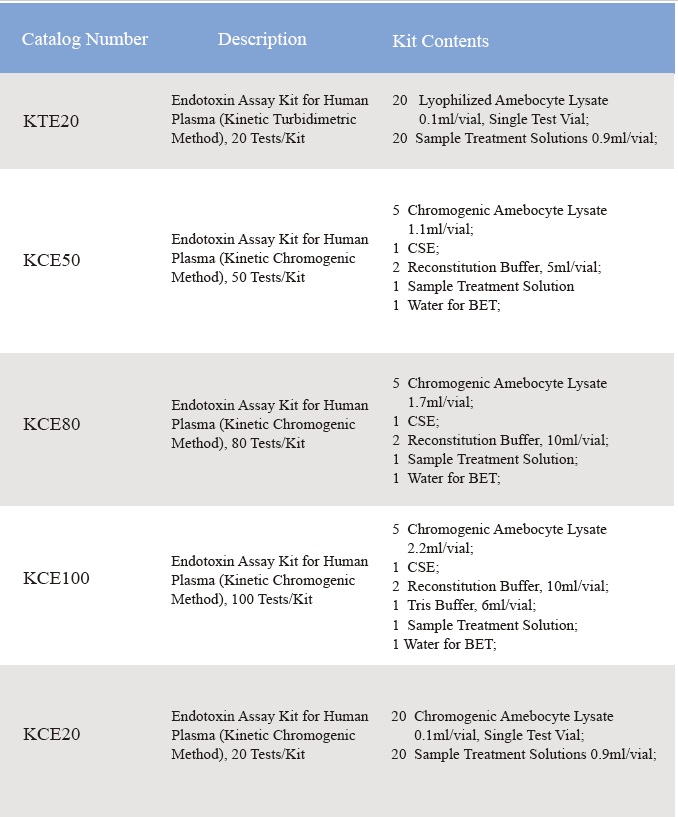
Uvakalelo lwe-Lyophilized Amebocyte Lysate kunye namandla okuLawula i-Endotoxin esemgangathweni ivavanywa ngokuchasene ne-USP Reference Standard Endotoxin.Iikiti ze-reagent ye-Lyophilized Amebocyte Lysate ziza nomyalelo wemveliso, iSatifikethi sokuHlalutya, i-MSDS.