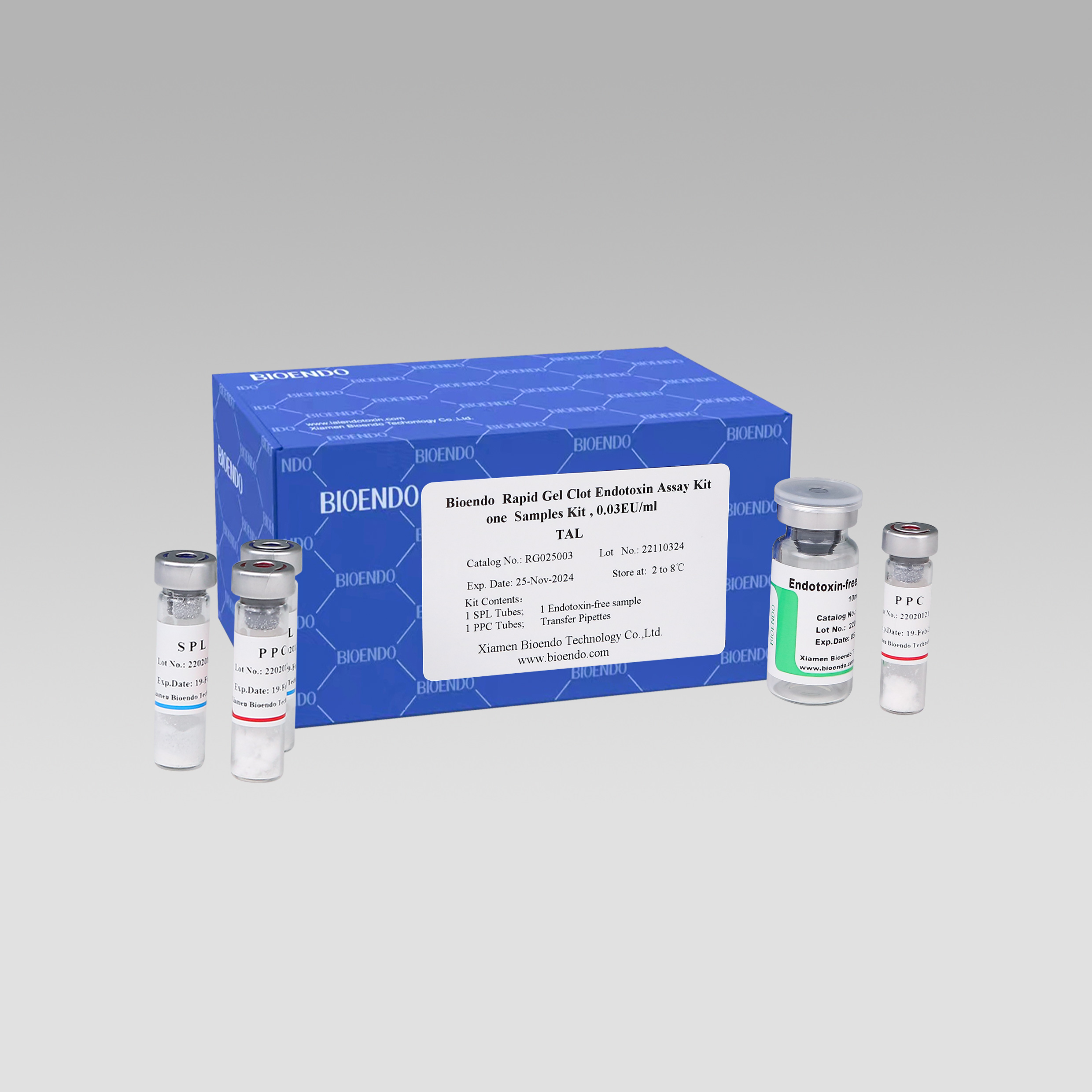
Ikhithi yovavanyo ye-Rapid Gel Clot enye
I-Rapid Gel Clot Endotoxin Test Kit (ISampuli enye yoVavanyo lweKhithi)
1. Ulwazi lweMveliso
I-Rapid Gel Clot Endotoxin Assay Kit yenzelwe ukulinganisa ngokukhawuleza i-endotoxin emanzini okanye i-dialysate.Ngokuqhelekileyo, funda umphumo malunga nemizuzu engama-30.
Phantsi kwesikhokelo sokubona i-endotoxin emanzini okanye i-dialysate ngokukhawuleza, ukubhaqwa kwe-endotoxin nge-Bioendo Rapid Gel Clot Endotoxin Assay Kit ayifuni ukuhlanjululwa kwamanyathelo amaninzi e-Endotoxin esemgangathweni kunye neesampuli zovavanyo.Iinkqubo ezilula neziluncedo ekusebenzeni kovavanyo olukhawulezayo lwe-endotoxin, wonke umsebenzi awufuni zixhobo zovavanyo ezinobunkunkqele, ukufakwa kwe-incubator yobushushu eyomileyo.Kuyafaneleka ngokukodwa uhlalutyo lwe-endotoxins emanzini okanye kwi-dialysate.
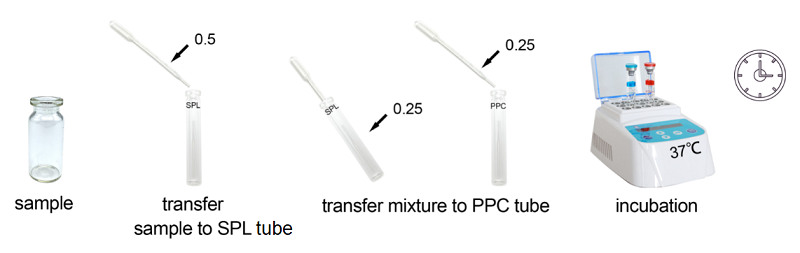
2. IParamitha yeMveliso
Uluhlu lweSensitivity: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5EU/ml
Uvavanyo olunye lwesampulu kwikhithi.
Ixesha lovavanyo: ngaphantsi kwemizuzu engama-30.
| Ikhathalogu No. | Inkcazo | IkhithiImixholo | Uvakalelo EU/ml | Ixesha lokuphendula imizuzu |
| RG025003 | I-BioendoTMI-Rapid Gel Clot Endotoxin Assay Kit, iSample Kit enye | I-Tube ye-SPL eyi-1; Umbhobho wePPC oyi-1; Ibhotile yeSampuli ye-Endotoxin-free; IiPipettors ezi-3 ezingenaPyrogen; | 0.03 | ≤60 |
| RG025006 | 0.06 | ≤60 | ||
| RG0250125 | 0.125 | ≤45 | ||
| RG025025 | 0.25 | ≤30 | ||
| RG025050 | 0.5 | ≤30 |
3. Isicelo seKit
Uvavanyo olulodwa lwe-Bioendo i-Rapid Gel Clot Endotoxin Assay Kit ibonelela ngohlobo lwesisombululo sovavanyo olukhawulezayo lwe-endotoxin, isicelo esibonakalisiweyo kwintsimi ye-dialysis.
Phawula:
I-reagent ye-Lyophilized Amebocyte Lysate (LAL) eyenziwe yi-Bioendo yenziwe nge-amebocyte lysate ethathwe kwigazi loononkala.Kwinkqubo yokusebenza, ukuxutywa komgangatho wolawulo we-endotoxin kulula kwaye kulula.I-endotoxin yesikhongozeli sasimahla sesampulu kunye ne-pyrogen yokudlulisa i-pipette yasimahla iyafuneka, isixhobo esiphucukileyo asifunwa, sincoma i-Bioendo Dry incubator ye-TAL-MT yokusetyenziswa kwindlela yokufukamela.
Ubumbeko lweKit:
Uvavanyo olulodwa lwe-Bioendo i-Rapid Gel Clot Endotoxin Assay Kit iqulethe:
Iqhekeza le-SPL Tube, i-1 ye-PPC Tube, iqhekeza le-Endotoxin-free Sample Bottle.(isampuli ye-depyrogenation cntainer enenqanaba eliphezulu),
1 Pack of Transfer Pipette 3 iziqwenga.(inqanaba eliphezulu leendotoxin)